GMP / cGMP
Good Manufacturing Practices
What is GMP / cGMP?
Good Manufacturing Practices is a system (much like a management system) that consists of processes, procedures, and documentation that ensures manufacturing food, cosmetics, cannabis products, and pharmaceutical goods are produced according to specific quality standards. GMPs can be implemented with ISO 9001 or separately.
The benefits of properly implementing GMPs can be:
- Reduced waste
- Safer food/consumable
- Little to no supply chain disruption
In the United States, the FDA (Federal Drug Administration) is responsible for setting the requirements for Good Manufacturing Practices (GMP) and Current Good Manufacturing Practices (cGMP). According to the FDA they “specify the minimum requirements for the methods, facilities, and controls used in manufacturing, processing, and packing of a drug product. The regulations make sure that a product is safe for use, and that it has the ingredients and strength it claims to have.”
cGMPs apply to manufacturers that must follow “Current” Good Manufacturing Practices, meaning their machines, technologies, and processes must conform to current requirements. The section below talks about those specific products that fall into the cGMP area.
Get a Free Quote
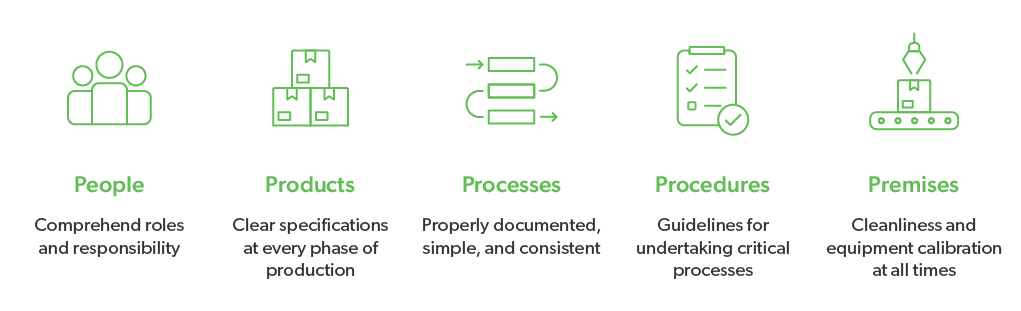
The 5 Ps of Good Manufacturing Practices
The 5 Ps of GMPs can be further broken down into these areas. Their purpose is to ensure safe, unadulterated food/consumable products:
- Quality management
- Sanitation and hygiene
- Building and facilities
- Equipment
- Raw materials
- Personnel
- Validation and qualification
- Complaints
- Documentation and recordkeeping
- Inspections and quality audits
The 5 P’s of Good Manufacturing Practices



The 5 Ps of GMPs can be further broken down into these areas. Their purpose is to ensure safe, unadulterated food/consumable products:
- Quality management
- Sanitation and hygiene
- Building and facilities
- Equipment
- Raw materials
- Personnel
- Validation and qualification
- Complaints
- Documentation and recordkeeping
- Inspections and quality audits
What is the FDA guidance relating to Cannabis Manufacturing?
The FDA does not have legal authority over Cannabis, but it does provide guidance for the Cannabis industry. Each state has its own legal and regulatory requirements. It is important to reference your state regulations. However, ensuring that cannabis products are produced safely, consistently, and with high quality is the goal of the FDA’s guidance.

Here are some important aspects of GMPs for Cannabis Manufacturers:
FDA Guidance: In accordance with the US Food and Drug Association FDA 21 CFR Part 111, Cannabis manufacturers should follow current good manufacturing practices (cGMPs). Cannabis products fall under the dietary supplements category.
Quality Control: Cannabis products must be produced under a quality control system that ensures their safety, identity, strength, quality, and purity. Implementing an ISO 9001 quality management system can assist in accomplishing these goals as well as implementing an effective cGMP system. Establishing a HACCP Plan is a great first step.
Facility Design and Control: The manufacturing facility should be designed and controlled to include proper handling, storage, and prevent contamination. cGMPs cover details relating to all aspects of this requirement.
Training and Competency: The organization needs to provide and have records of appropriate training of staff and demonstration of their competency to produce quality products according to specifications.
Labeling: All cannabis products must comply with applicable labeling requirements, including listing all ingredients and making truthful and non-misleading claims about the product.
Product Testing: Cannabis manufacturers are asked to have 3rd party testing of their products; however, it is sometimes cost-prohibitive to test every batch and lot, especially with a 3rd party. Documentation of test results is important to show that the products are manufactured to specification and meet label claims.
Traceability: Maintaining proper records of lots and batches from seed to final product is critical for lot trace and is important in the chance of a recall.
Documentation: Any good management system includes appropriate documentation of Procedures, Policies, Testing Results, Batch Records, Training, and other relevant records.
The purpose behind implementing cGMPs and/or an ISO 9001 quality management system is to reduce the risk of recall, ensure the purity of products, and consistency in producing products according to specifications and labeling claims. Many organizations may not have the money for certification; however, you can implement and maintain compliance without becoming certified.

When it comes to pharmaceutical products or biotech products, the cGMPs apply as do the following FDA regulations depending on your product(s):
- 21CFR Part 314: FDA Approval to market a new drug
- 21CFR Part 210: Processing, Packing, or Holding of Drugs
- 21CFR Part 211: Finished Pharmaceuticals
- 21CFR Part 600: Biological Products
Related Standards
We provide consulting support for various other standards, as well as support for companies seeking multiple certifications through an Integrated Management System.
ISO 9001
Quality Management Systems
ISO 17025
Testing, Sampling, or Calibration
ISO 9001
ISO 17025
Core Business Solutions offers consulting services for GMP compliance and GMP certification. If you’re involved in the manufacture, testing, or transportation of cosmetics, your customers and partners expect a high level of safety and quality – the ISO 22716 standard would apply to you. To read more about ISO 22716 check out our article.For more information about GMP or cGMP, please call our consulting office at 866-354-0300 or contact us online.